The ORBITA trial: what it means to the Interventional Cardiologist
Jie Li Kua11, Joshua P. Loh2
1Ng Teng Fong General Hospital, Singapore
2National University Heart Centre, Singapore
Introduction
The concept of angioplasty for the treatment of severe coronary artery stenosis originated in 19771. Since then, angioplasty has evolved from plain old balloon angioplasty to bare metal stents and now contemporary 2nd generation drug eluting stents. Major guidelines worldwide, including the American College of Cardiology/ American Heart Association and the European Society of Cardiology for stable ischemic heart disease (ACC/AHA) or chronic coronary syndrome (ESC) have since recommended revascularization as a treatment modality for angina when there is failure of response to optimal medical therapy2,3. The authors of ORBITA argue that previous trials demonstrating relief of angina from angioplasty in which the guideline recommendations could be related to a placebo effect, and therefore this places the importance of finding the true efficacy of percutaneous coronary intervention (PCI)4. Hence, ORBITA was designed as a trial to compare angioplasty to placebo using an objective outcome measure of treadmill exercise time5.
ORBITA trial design and results
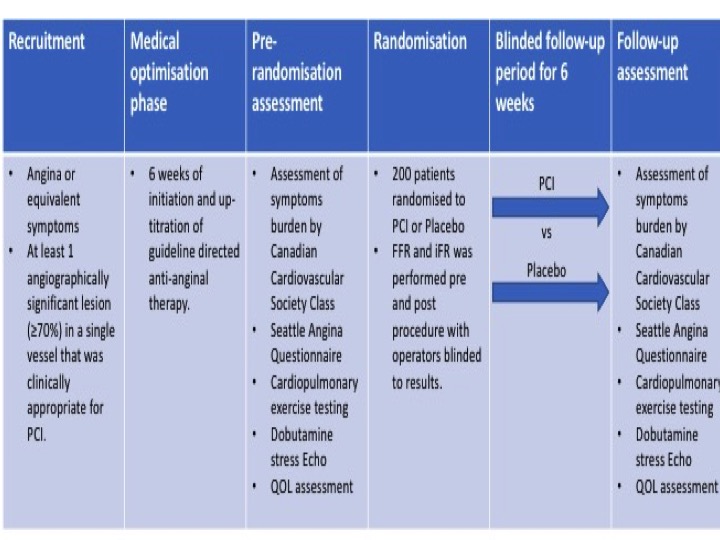
ORBITA was a double blind, randomised trial done at 5 study sites in the UK (Figure)5. Percutaneous coronary intervention was compared to placebo for angina relief in patients with severe (≥ 70%) stenosis. Between 18th December 2013 and 26th July 2017, 368 patients with angina and single vessel coronary disease were assessed for eligibility with 230 eventually being enrolled. After enrolment, medications were introduced and up-titrated aiming for at least two antianginal therapies per patient during a 6-week period. After the medical optimisation phase, cardiopulmonary exercise testing, symptom questionnaires and dobutamine stress echocardiography were performed as part of pre-randomization assessments. Randomised blinded procedure was then performed including measurements of both fractional flow reserve (FFR) and instantaneous wave free ratio (iFR) for research purposes. The clinical operator was blinded to the physiology values and therefore did not use them to guide treatment. 200 patients were sedated and underwent randomisation to PCI versus placebo. In the next 6 weeks of follow-up, assessments done before the procedure were repeated at the final assessment. The primary end point was difference in exercise time increment between groups. The results of the trial found that lesions had a mean area stenosis of 84.5% (SD 10.2) with mean physiologic measurements of FFR 0.69 (0.16) and instantaneous wave-free ratio of 0.76 (0.22). There was no significant difference in the primary endpoint of exercise time increment between groups (PCI minus placebo 16.6 s, 95% CI -8.9 to 42.0 p=0.200). There were no deaths but 4 pressure-wire related complications happened in the placebo group which required PCI and five major bleeding events. The authors conclude that in patients with medically treated angina and severe coronary stenosis, PCI did not improve exercise time by more than the effect of a placebo procedure.
Critique of the ORBITA trial
The results of ORBITA caused an uproar in the cardiology community. Multiple editorials have been published mostly criticising the trial6-10. These are some of the criticisms.
- In the ORBITA trial, medications were much better optimised as compared to reality. Patients were up-titrated to an average of three anti-anginal agents during the initial 6 weeks before randomisation. They had telephone consultations once to three times a week with a consultant cardiologist. This intense follow-up period is impractical. Also, getting patients to such high doses of medications is also likely to have blunted the treatment effect.
- The patients in the PCI group started with an exercise time of 528 seconds while those in the placebo group started with 490 seconds. This means that patients were already capable of exercising to stage 3 on the treadmill prior to procedure. The Seattle Angina Questionnaire for physicial limitation was 71.3 in the PCI group and 69.1 in the placebo group pre-randomisation. For comparisons, the COURAGE trial patients had a baseline SAQ score of 66 which rose to 76 in the PCI group and 72 in the medical therapy group11. The impact of PCI on this group of patients with mild limitation in effort tolerance is unlikely to be significant.
- It is a class 1 indication that invasive functional assessment must be available and be used to evaluate stenoses before revascularization2. Amongst the study patients, 57 (29%) had FFR greater than 0.80 and 64 (32%) had iFR greater than 0.89. These numbers form almost a third of the total number of patients and would likely have blunted the actual benefit of angioplasty on truly ischemic lesions.
Proponents however applaud the authors for conducting a blinded PCI trial which is the first of its kind and also for the rigour of their trial7,12. Sham procedure trials are extremely rare13. The most notable one in recent times was the SYMPLICITY trial where renal denervation did not prove its expected benefit in poorly controlled hypertension despite previous promising results. There has never been a placebo controlled trial done on PCI which makes the ORBITA trial not only remarkable but also a landmark trial.
In an editorial response, Rasha et al have addressed some criticisms and highlighted challenges they faced with the conduct of their trial14. She explained in the editorial that ORBITA was designed to detect a 30 second effect size beyond placebo and this only required 200 patients hence it was not justified to recruit more patients unless they were looking at a shorter effect size. The short duration of follow-up post PCI was also a balance as the patients recruited had angiographically significant lesions therefore leaving them on medical therapy may incur a higher risk.
The same group also published a physiology-stratified analysis of ORBITA15. The report describes how blinded iFR and FFR (which was obtained pre-randomisation) predict placebo-controlled effect of PCI on stress echocardiography score, patient-reported and physician assessed symptoms, quality of life and treadmill exercise time. As the primary analysis did not adjust for between-group differences, regression models were used for this physiology-stratified analysis instead. They found that the estimated effect of PCI on between-arm prerandomization-adjusted total exercise time was 20.7 seconds (95% CI, -4.0 to 45.5, P=0.100) with no interaction of FFR or iFR. PCI improved stress echocardiography more than placebo (1.07 segment units; 95% CI, 0.70 – 1.44; P<0.00001). The placebo-controlled effect of PCI on stress echocardiography score increased progressively with decreasing FFR and iFR. Although PCI did not improve angina frequency score significantly more than placebo, it did result in more patient-reported freedom from angina than placebo (49.5% versus 31.5%; odds ratio, 2.47; 95% CI, 1.30-4.72; P=0.006). Neither FFR nor iFR modified this effect.
Following the initial buzz, major guidelines have not changed with regards to their recommendations for revascularization. In the ESC guidelines, a brief mention was made of the ORBITA trial2. The study highlights a significant placebo component to the clinical effects and alerts us to pitfalls of interpreting end-points subject to bias in the absence of sham control and blinding. However, due to the limited trial size, short-term observation time until crossover and insufficient power to assess clinical end points, the results cannot change guidelines.
Significance of the ORBITA trial to the Interventional Cardiology community
The conduct of the ORBITA trial is the first of its kind and tries to address a question that has never been answered before; does PCI improve angina as compared to placebo. The belief in angioplasty and its role in the treatment for angina has been so ingrained in the cardiology community that the results of ORBITA took us all by surprise. Given the small number of patients in the trial, it maybe premature to draw any definitive conclusion from the study results at this point in time. However, the results should certainly encourage us to rethink our understanding of the underlying pathophysiology of angina. The simplified concept is that a stenosis in a vessel impedes flow which in turn causes ischemia to myocardial tissue and hence symptoms of angina. The expectation is the resolution of this stenosis through angioplasty would therefore relieve the angina. Perhaps this maybe an oversimplified way of looking at it16.
While there are still many uncertainties that looms around the topic, the big question is whether the interventional community will be receptive to more negative results. Unlike in acute coronary syndromes, the evidence for angioplasty in stable ischemic lesions has not been as robust. The more recently concluded ISCHEMIA trial was yet another blow to the invasive treatment of stable ischemic heart disease17. In this trial, patients with moderate to severe ischemia were randomized to invasive coronary angiography followed by revascularization if needed, on top of optimal medical therapy or to an initial conservative strategy of optimal therapy alone. Randomization was done prior to angiography and blinded CT angiography was performed in approximately two thirds of the enrolled cohort to exclude life-threatening left main disease. Over a period of 3.2 years, 318 primary outcome events occurred in the invasive strategy group and 352 occurred in the conservative strategy group. At 6 months, the cumulative event rate was 5.3% in the invasive strategy group and 3.4% in the conservative strategy group (difference 1.9 percentage points; 95% confidence interval, 0.8 to 3.0). At 5 years, the cumulative event rate was 16.4% and 18.2% respectively (difference, -1.8 percentage points; 95% CI, -4.7 to 1.0).
Guidelines have recommended PCI as an option when there is failure of medical therapy. Yet angioplasty is often performed for patients have not yet been fully optimised on medications18. This is often driven both by the clinician as well as the patient who feels safer having their artery fixed. The results of ORBITA reinforces the notion that medications are key in angina management. It is not as yet the final nail in the coffin of PCI but with the summation of recent evidence, it should not be a surprise if we are indeed heading in that direction.
Given the recent evidence, patient participation in the decision-making process is ever more important. Not all cardiac patients may favor having a stent. Leaving permanent metal in their bodies and having to take 2 blood thinning medications for a prolonged period of time are just some deterrences. This is in line with the global trend towards patient centred medicine where patients now taking an equally important role in deciding what therapeutic option would be best for themselves19.
Conclusion and future directions
The ORBITA investigators have now embarked on their “ORBITA Part 2” (NCT03742050). This will be a 400-patient trial recruiting patients with angina, significant coronary stenosis as noted from their coronary angiogram or CT angiography and also demonstrating evidence of ischemia. They are randomized to percutaneous coronary intervention or placebo, looking at change in angina symptom score as an outcome measure at 12 weeks. Recruitment started in November 2018 and is expected to end in November 2022. They have addressed 2 major critiques of the original trial. Only those with objective evidence of ischemia (whether invasive or non-invasive) will be studied. The length of follow-up has also been extended to 12 weeks. This will answer the important question of whether angina improves with PCI when compared to placebo without any background angina therapy. I look forward to the results of ORBITA2 and even more so the response from the interventional community when the results are published.
The ORBITA trial, the only randomized double-blind placebo controlled trial enrolling patients with stable angina failed to demonstrate improvements in exercise time at 6 weeks in patients treated with PCI compared to a placebo procedure. While this has lead to much debate in the interventional community, it has given us new perspectives to rethink our treatment approach of stable ischemic heart disease and also opened up new horizons in the conduct of future trials.
Figure: ORBITA Trial design
References
- Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1(8058):263.
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):2564-2603.
- Francis DP, Al-Lamee R. Percutaneous coronary intervention for stable angina in ORBITA - Authors' reply. Lancet. 2018;392(10141):28-30.
- Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial [published correction appears in Lancet. 2018 Jan 6;391(10115):30]. Lancet. 2018;391(10115):31-40. doi:10.1016/S0140-6736(17)32714-9
- Chaitman BR, Mori Brooks M, Fox K, Lüscher TF. ORBITA revisited: what it really means and what it does not?. Eur Heart J. 2018;39(11):963-965.
- Dickert NW, Miller FG. Sham-Controlled Trials for Coronary Interventions: Ethically Acceptable and Ethically Important. J Am Coll Cardiol. 2018;71(1):95-97.
- Schueler R, Al-Lamee R, Mahfoud F, Capodanno D, Al Asnag M, Haude M. Will ORBITA change my practice? ORBITA trial: Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina. EuroIntervention. 2018;14(8):951-954.
- Byrne RA. Fallout from the ORBITA trial - is angioplasty in a spin?. EuroIntervention. 2017;13(11):1253-1254.
- Kirtane AJ. ORBITA2. Circulation. 2018;138(17):1793-1796.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359(7):677-687.
- Brown DL, Redberg RF. Last nail in the coffin for PCI in stable angina?. Lancet. 2018;391(10115):3-4.
- Redberg RF. Sham controls in medical device trials. N Engl J Med. 2014;371(10):892-893.
- Al-Lamee R, Francis DP. Swimming against the tide: insights from the ORBITA trial. EuroIntervention. 2017;13(12):e1373-e1375.
- Al-Lamee R, Howard JP, Shun-Shin MJ, et al. Fractional Flow Reserve and Instantaneous Wave-Free Ratio as Predictors of the Placebo-Controlled Response to Percutaneous Coronary Intervention in Stable Single-Vessel Coronary Artery Disease. Circulation. 2018;138(17):1780-1792.
- Mangiacapra F, Del Buono MG, Abbate A, et al. Role of endothelial dysfunction in determining angina after percutaneous coronary intervention: Learning from pathophysiology to optimize treatment [published online ahead of print, 2020 Feb 13]. Prog Cardiovasc Dis. 2020;S0033-0620(20)30039-6.
- Maron DJ, Hochman JS, Reynolds HR, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382(15):1395-1407.
- Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention [published correction appears in JAMA. 2011 Jun 15;305(23):2418]. JAMA. 2011;305(18):1882-1889.
- Bardes CL. Defining "patient-centered medicine". N Engl J Med. 2012;366(9):782-783.
