Percutaneous Coronary Intervention in Small Vessel Coronary Artery Disease: A Contemporary Approach
Authors
Hui Wen Sim, Joshua Loh
National University Heart Centre, Singapore
Abstract
Small vessel disease (reference vessel diameter ≤ 2.8mm) is an important risk factors for adverse outcome after percutaneous coronary intervention. Late lumen loss is always a concern with stenting due to a combination of stent struts and neointimal hyperplasia, even with newer generation drug eluting stents. In the contemporary era, drug-coated balloon is emerging as an effective alternative treatment for small vessel disease. Studies comparing drug coated balloons and drug eluting stents in small vessel disease had shown mixed results. To date, there is still no consensus on the optimal strategy for treating this subset of coronary anatomy. This present article provides an updated review on percutaneous coronary intervention in small vessel disease.
Abbreviations
BMS: bare metal stents; DCB: drug coated balloons; DES: drug eluting stents; PCI: percutaneous coronary intervention; MACE: major adverse cardiovascular events; RVD: reference vessel diameter; SVD: small vessel disease.
Background
Small vessel disease (SVD), defined as reference vessel diameter (RVD) of ≤ 2.8mm, is commonly seen in up to 30-50% of all percutaneous coronary intervention (PCI)1,2. SVD an important risk factors for adverse outcome after PCI1,2. Smaller calibre coronary arteries have limited ability to accommodate late lumen loss from stent struts and neointimal hyperplasia as compared to larger vessels. There is a growing interest in this topic in the recent years but there is still lack of consensus on the optimal treatment in this group of patients.
Pre-DES era PCI of SVD
In the earlier days, the options of treating SVD include plain balloon angioplasty and bare metal stents (BMS). The use of plain balloon angioplasty was associated with high risk of restenosis. The major drawbacks of plain balloon angioplasty were early elastic recoil, arterial remodelling after dilatation and neointimal hyperplasia. The ISAR-SMART study (n=404) was the first randomized trial to compared plain balloon angioplasty and BMS in SVD. At 7 months, there was no difference in the angiographic restenosis rate for plain balloon angioplasty and BMS (37.4% versus 35.7%, p=NS)3. The acute lumen gain after stenting may be offset by late lumen loss during follow up, causing similar outcomes in both groups of patients. Conversely, subsequent studies and meta-analysis demonstrated advantage of stenting over plain balloon angioplasty in reducing restenosis. In the large-scale multicentre BESMART trial (n=381), angiographic restenosis rates and repeat revascularization rates at 6 months were lower in the BMS group as compared to plain balloon angioplasty (angiographic restenosis: 21% vs 47%, p<0.001, target lesion revascularization: 13% vs 25%, p<0.001)4. Subsequently, a meta-analysis (n=4383) comparing plain balloon angioplasty and BMS found that major adverse cardiovascular events (MACE), mainly driven by repeat revascularization were lower for BMS group (17.6% vs 22.7%, Odds ratio 0.71, 95% CI: 0.57-0.90)5. However, the authors warned against substantial heterogenicity of the trials included with high bailed-out stenting rates (22%) in the plain balloon angioplasty group.
First- and second-generation DES
First generation drug eluting stents (DES) was shown to be superior to BMS in preventing restenosis by eluting anti-proliferative drugs6. In the SVD group of the TAXUS V randomized trial (n=1156), angiographic restenosis and target vessel revascularization rates at 9 months was lower in those receiving paclitaxel DES as compared to BMS (angiographic restenosis: 31.2% vs 49.4%, p=0.01; target lesion revascularization: 10.4% vs 21.5%, p=0.03)7. Real-world data from the single arm paclitaxel DES TRUE registry (n=675) also demonstrated acceptable 1-year MACE (17.3%), repeat revascularization (12.8%) and stent thrombosis rates (0.9%) as compared to historical BMS cohort8. Despite showing better results than BMS, there is still concerns regarding restenosis, late and very late stent thrombosis for first-generation DES in relation to stent design, presence of durable polymer and delayed vascular healing9. Second-generation DES were developed to overcome the limitation of first-generation DES with more potent antiproliferative agents, improved polymer and thinner fracture-resistant struts. In the SPIRIT SV “small vessel” single arm registry (n=150), everolimus DES demonstrated acceptable revascularization rate (5.1%) and stent thrombosis rate (1.5%) at 1 year10. The BASKET-SMALL pilot study randomized 191 patients with SVD to first generation paclitaxel DES versus second generation zotarolimus DES. After 2 years, revascularization rates were numerically higher in the in the paclitaxel DES arm (6.6%) than zotarolimus DES arm (2.0%), but the study was underpowered to detect a difference in the event rates11.
Drug-coated balloon in SVD
Drug-coated balloon (DCB) is emerging as an effective alternative treatment for SVD. Compared to DES, DCB allows for easier delivery of device, broader and more homogenous surface contact for drug transfer, higher drug concentration, absence of stent strut and long-term polymer that poses risk of thrombosis and allows for shorter duration of dual antiplatelet therapy. The PEPCAD-1 study (n=118) was the first to study the outcome of DCB in SVD. In this single arm observational study of 118 patients treated with paclitaxel DCB, MACE and revascularization rate at 1 year was 15% and 12%, respectively12. Several studies subsequently compared clinical outcomes between paclitaxel DCB and first-generation paclitaxel DES in SVD and found mixed results. The PICCOLETTO trial (n=57) was the first randomized study to compare Dior-I paclitaxel DCB to paclitaxel DES. They failed to show equivalence of paclitaxel DCB to paclitaxel DES with regards to angiographic end points. In fact, the study was terminated prematurely due to superiority in the DES group13. It was postulated that geographical miss and poor lesion preparation were among the reasons for poor results in the DCB group. Subsequently, the BELLO trial assigned 182 patients to INPACT Falcon paclitaxel DCB or paclitaxel DES and reported lower MACE rate in the DCB group at 3 years follow up (15.4% vs. 38.9%, p=0.02)14. However, there was no difference in the revascularization rate which questioned if the study were underpowered to detect any differences. Following that, studies comparing paclitaxel DCB with second-generation DES also yield mixed results. The BASKET-SMALL 2 open-label randomized study (n=758) found that paclitaxel DCB was non-inferior to paclitaxel or everolimus DES at 1 year follow up (MACE: 7.5% for DCB vs 7.3% for DES, p=0.918)15. In contrast, a recently published report from the Swedish Coronary and Angioplasty Registry (SCAAR) with the largest number of patients compared the clinical outcomes of SVD treated with paclitaxel DCB (n=1154, SeQuent Please, IN.PACT Falcon and Pantera Lux) or newer-generation DES (n=13,634, Resolute, Xience, Promus, Synergy, Orsiro, Biomatrix and Ultimaster)16. A propensity score-adjusted regression analysis showed a higher risk of restenosis rates (based on angiographic restenosis or functional ischemia by fractional flow reserve) in the DCB group as compared to the DES group at 3 years follow up (4.1% vs 1.8%, adjusted hazard ratio 2.027, 95% CI 1.537 – 2.674). There was no difference in lesion/stent thrombosis, myocardial infarction and death. This results from this large registry suggest that paclitaxel DCB may not be as effective as newer-generation DES in treating SVD. This is hypothesis generating and further confirmatory randomized trials that are adequately powered are still needed to decide on the best strategy for SVD. Sirolimus DCB is the latest device in the market using encapsulated nanocarrier to deliver antiproliferative drug during balloon angioplasty. The single arm Nanoluté registry using Magic Touch sirolimus DCB with 156/332 SVD patients demonstrated an overall acceptable target lesion revascularization rate of 3.6% at 1 years17. Similarly, further dedicated clinical trials in SVD are needed to study the safety and efficacy or this novel device.
PCI in very small vessels
PCI of very small vessels (2mm) is technically challenging. It is usually a manifestation of a diffuse disease process; therefore, a higher rate of repeat revascularization could be anticipated. In very small vessel disease (RVD 2.0-2.25 mm), zotaralimus DES demonstrated a low rate of target lesion revascularization of 2.0% with no stent thrombosis at 1 year18. Furthermore, in a high risk all-comers population that included 7.0% cardiogenic shock patients, there was no difference in the target lesion revascularization or stent/lesion thrombosis rate for patients treated with 2.0mm DES versus 2.0mm paclitaxel DCB19. However, DCB technology is still evolving and studies have yet to show the superiority of one strategy over the other. Further large-scale clinical trials are warranted to make a decision on the preferred option for treating SVD.
A contemporary approach
Optimal lesion preparation is essential in using DCB as treatment strategy. A reasonable approach would be to re-evaluate the lesion after plain balloon angioplasty. The German Consensus group recommended that lesions in small vessels should be pre-dilated with balloon/vessel ratio of 0.8-1.020. The DCB should be extended beyond the pre-dilated segment by 2 to 3 mm on either end and inflated at nominal pressure for at least 30 seconds. A DCB may be the preferred option if the result after balloon angioplasty is optimal. If result after plain balloon angioplasty is suboptimal (major dissection, TIMI <III or residual stenosis >30%), the option of implanting thin-strut DES is available. (Figure 1)
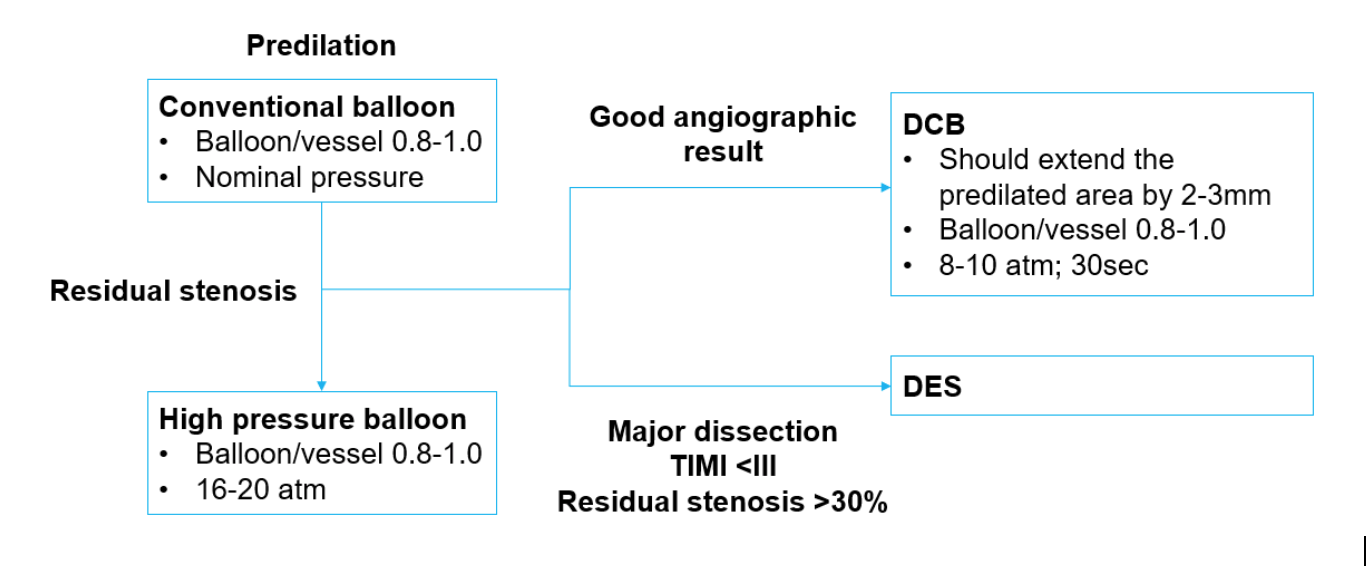
Figure legends
Figure 1: Treatment recommendation from the German Consensus Group for small vessel disease (reference vessel diameter 2.25 – 2.75mm)
DCB: drug coated balloon; DES: drug eluting stent
Adapted from: Kleber FX, Mathey DG, Rittger H, Scheller B; German Drug eluting Balloon Consensus Group. How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention. 2011;7:125-128.
References
- Foley DP, Melkert R, Serruys PW. Influence of coronary vessel size on renarrowing process and late angiographic outcome after successful balloon angioplasty. Circulation. 1994;90(3):1239-1251.
- Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schömig A. Vessel size and long-term outcome after coronary stent placement. Circulation. 1998;98(18):1875-1880.
- Kastrati A, Schomig A, Dirschinger J, Mehilli J, Dotzer F, von Welser N, Neumann FJ. A randomized trial comparing stenting with balloon angioplasty in small vessels in patients with symptomatic coronary artery disease. Circulation. 2000;102(21):2593-2598.
- Koning R, Eltchaninoff H, Commeau P, Khalife K, Gilard M, Lipiecki J, Coste P, Bedossa M, Lefèvre T, Brunel P, Morice MC, Maillard L, Guyon P, Puel J, Cribier A; BESMART (BeStent in Small Arteries) Trial Investigators. Stent Placement Compared With Balloon Angioplasty for Small Coronary Arteries. Circulation. 2001;104(14):1604-1608.
- Agostoni P, Biondi-Zoccai GGL, Gasparini GL, Anselmi M, Morando G, Turri M, Abbate A, McFadden EP, Vassanelli C, Zardini P, Colombo A, Serruys PW. Is bare-metal stenting superior to balloon angioplasty for small vessel coronary artery disease? Evidence from a meta-analysis of randomized trials. Eur Heart J. 2005;26(9):881-889.
- Hockenhull J, Greenhalgh J, Dickson RC, Ricciardi M, Patel A. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev. 2015;2015(10).
- Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, O'Shaughnessy CD, DeMaio S, Hall P, Popma JJ, Koglin J, Russell ME; TAXUS V Investigators. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. Jama. 2005;294(10):1215-1223.
- Godino C, Furuichi S, Latib A, Morici N, Chieffo A, Romagnoli E, Tamburino C, Barbagallo R, Cera M, Antoniucci D, Goktekin O, Di Mario C, Reimers B, Grube E, Airoldi F, Sangiorgi GM, Colombo A. Clinical and Angiographic Follow-Up of Small Vessel Lesions Treated With Paclitaxel-Eluting Stents (from the TRUE Registry). Am J Cardiol. 2008;102(8):1002-1008.
- Joner M, Finn A V., Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of Drug-Eluting Stents in Humans. Delayed Healing and Late Thrombotic Risk. J Am Coll Cardiol. 2006;48(1):193-202.
- Cannon LA, Simon DI, Kereiakes D, Jones J, Mehran R, Kusano H, Zhang Z, Lombardi W, James Fleischhauer F, Costa MA. The XIENCE nano everolimus eluting coronary stent system for the treatment of small coronary arteries: the SPIRIT Small Vessel trial. Catheter Cardiovasc Interv. 2012;80(4):546-553.
- Jeger R, Pfsterer M, Pfster O, Rickenbacher P, Handke M, Gilgen N, Coslovsky M, Kaiser C. First-generation paclitaxel- vs. second-generation zotarolimus-eluting stents in small coronary arteries: The BASKET-SMALL Pilot Study. Postep w Kardiol Interwencyjnej. 2016;12(4):314-320.
- Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H, Boxberger M, Degenhardt R, Scheller B. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99(3):165-174.
- Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, Limbruno U. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart. 2010;96(16):1291-1296.
- Latib A, Ruparelia N, Menozzi A, Castriota F, Micari A, Cremonesi A, De Felice F, Marchese A, Tespili M, Presbitero P, Sgueglia GA, Buffoli F, Tamburino C, Varbella F, Colombo A. 3-Year Follow-Up of the Balloon Elution and Late Loss Optimization Study (BELLO). JACC Cardiovasc Interv. 2015;8(8):1132-1134.
- Jeger R V., Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, Weilenmann D, Wöhrle J, Richter S, Schreiber M, Mahfoud F, Linke A, Stephan FP, Mueller C, Rickenbacher P, Coslovsky M, Gilgen N, Osswald S, Kaiser C, Scheller B; BASKET-SMALL 2 Investigators. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849-856.
- Silverio A, Buccheri S, Venetsanos D, Alfredsson J, Lagerqvist B, Persson J, Witt N, James S, Sarno G. Percutaneous Treatment and Outcomes of Small Coronary Vessels: A SCAAR Report. JACC Cardiovasc Interv. 2020;S1936-8798(19):32476-32478.
- Dani S, Shah D, Sojitra P, Parikh K, Shetty R, di Palma G, Cortese B. A novel nanocarrier sirolimus-coated balloon for coronary interventions: 12-Month data from the Nanoluté Registry. Cardiovasc Revascularization Med. 2019;20(3):235-240.
- Price MJ, Saito S, Shlofmitz RA, Spriggs DJ, Attubato M, McLaurin B, Popma Almonacid A, Brar S, Liu M8, Moe E, Mehran R. First Report of the Resolute Onyx 2.0-mm Zotarolimus-Eluting Stent for the Treatment of Coronary Lesions With Very Small Reference Vessel Diameter. JACC Cardiovasc Interv. 2017;10(14):1381-1388.
- Sim HW, Ananthakrishna R, Chan SP, Low AF, Lee CH, Chan MY, Tay EL, Loh PH, Chan KH, Tan HC, Loh JP. Treatment of Very Small De Novo Coronary Artery Disease With 2.0 mm Drug-Coated Balloons Showed 1-Year Clinical Outcome Comparable With 2.0 mm Drug-Eluting Stents. J Invasive Cardiol. 2018;30(7):256-261.
- Kleber FX, Mathey DG, Rittger H, Scheller B. How to use the drug-eluting balloon: Recommendations by the German consensus group. EuroIntervention. 2011;7(SUPPL. K):125-128.
